Heartwarming Info About How To Write An Ionic Compound

Write formulae of ionic compounds.
How to write an ionic compound. This is so that the charges are balanced and the compound is neutral. 14k views 11 years ago. Writing formulas for ionic compounds containing polyatomic ions.
Learn how to name monatomic ions and ionic compounds containing monatomic ions, predict charges for. Recognize polyatomic ions in chemical formulas. Molecules, compounds and chemical equations.
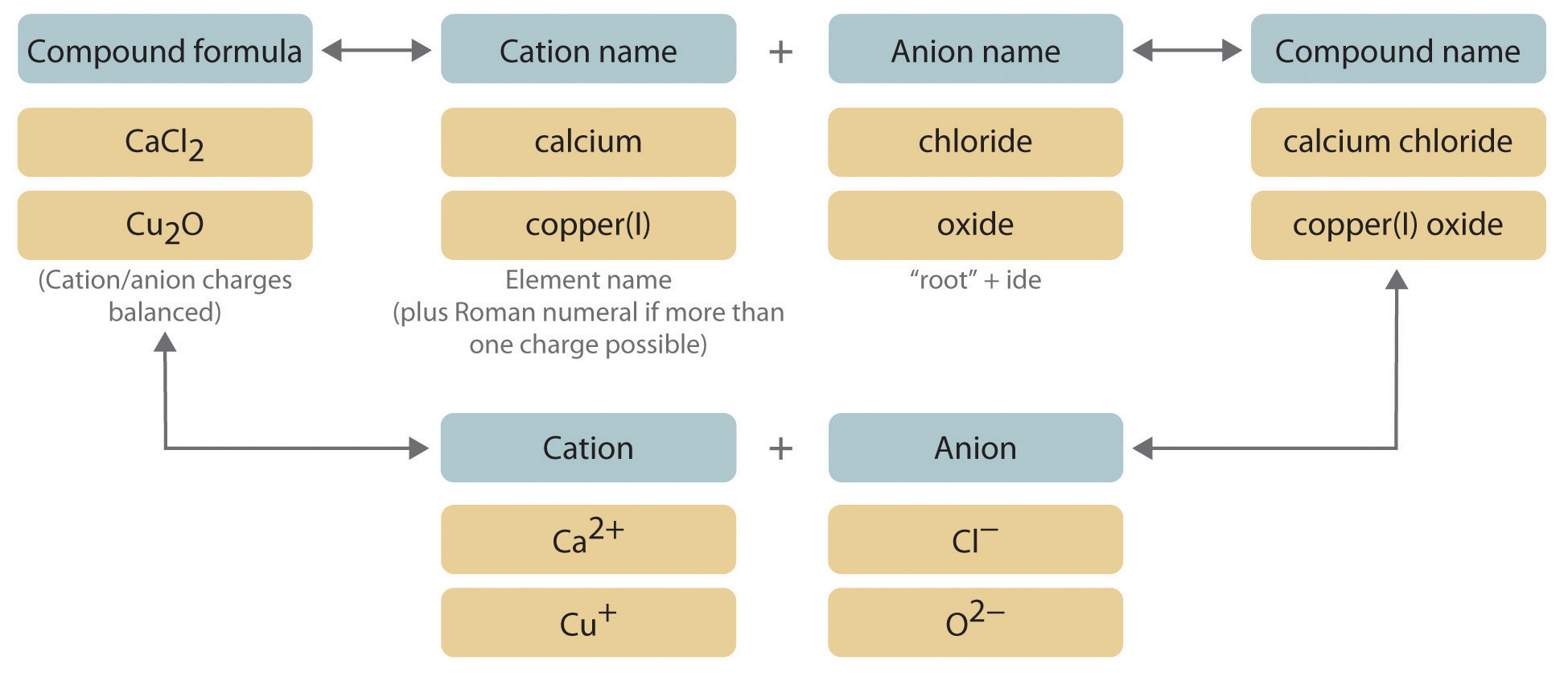
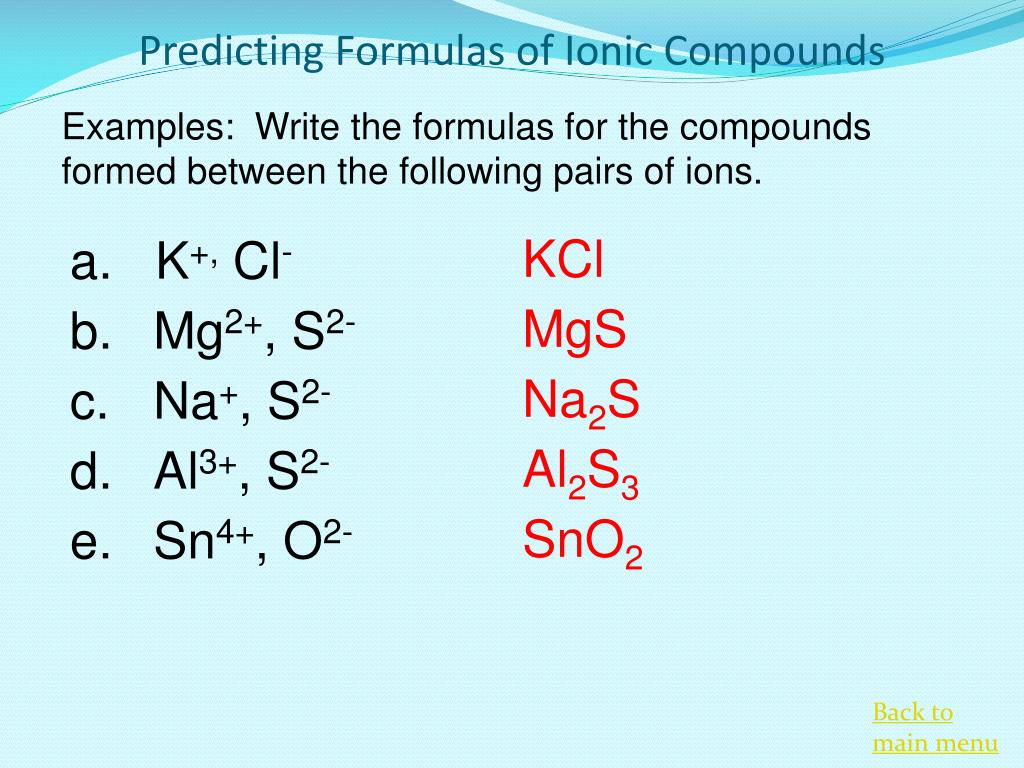
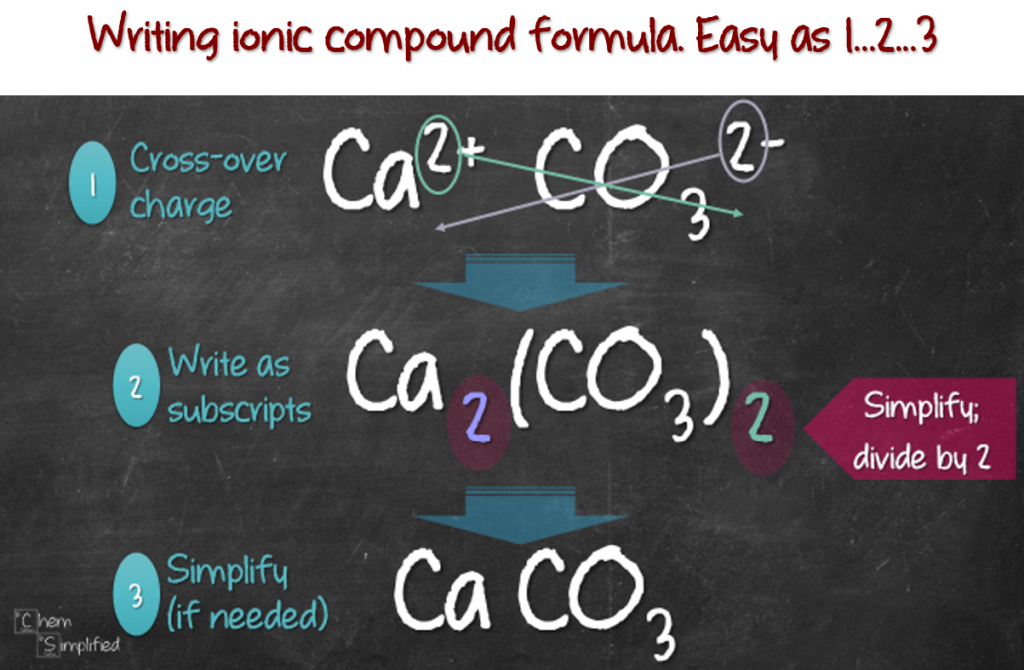
To write a chemical formula of an ionic compound, the charges of the ions must be determined first. This page explains how to work out. Write the correct formula for an ionic compound.
Writing formulas for ionic compounds containing polyatomic ions. A molecular approach (tro) 3: Ionic compounds do not exist as molecules.
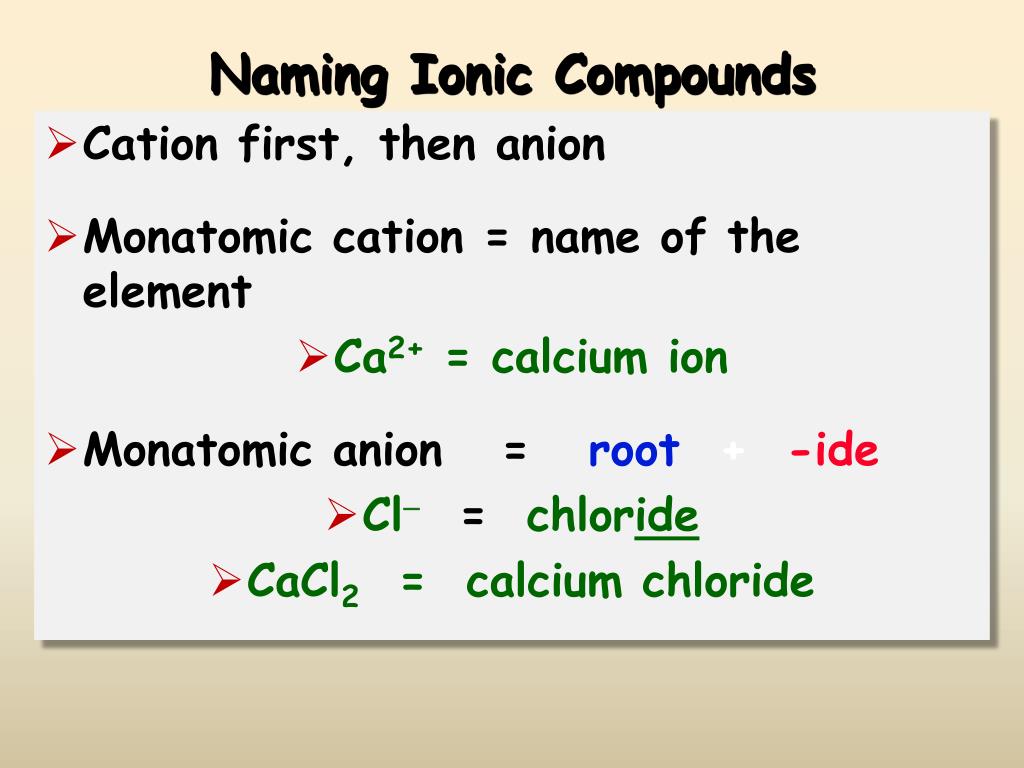
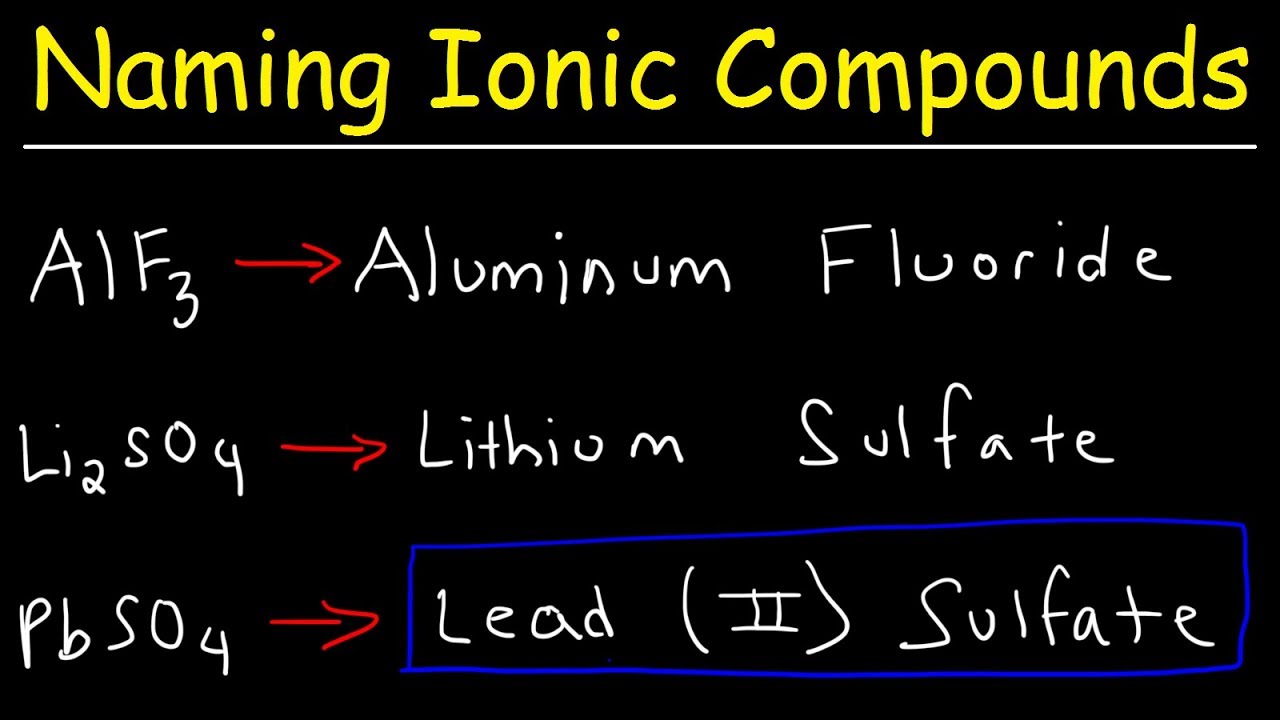
How to write formulae for simple ionic compounds. Naming monatomic ions and ionic compounds. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge.
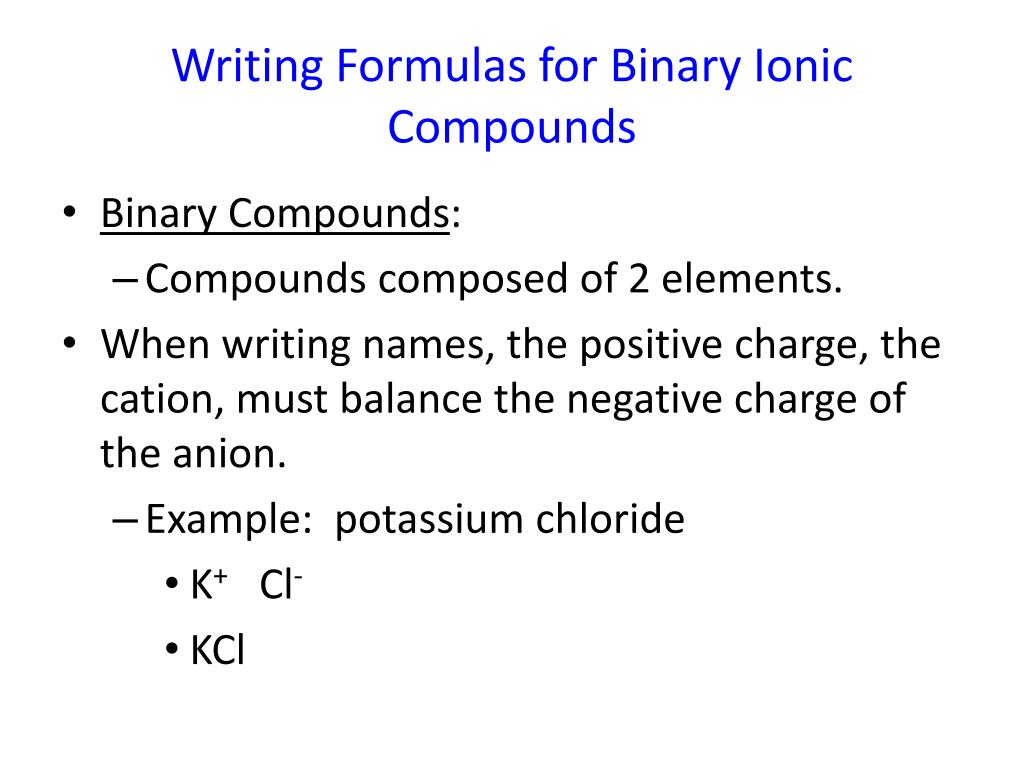
Here's how to write formulas for binary ionic. Give each student a set of ’traffic light’ cards. This chemistry video tutorial provides an introduction to writing the formula of an ionic compound that contains transition metals with roman numerals and po.
How to write formulae for simple ionic compounds. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The formula for an ionic compound must contain the same number of positive and negative.
The charge of the cation becomes the subscript of the anion,. Then, identify the anion and write down its symbol and charge. Writing a formula for ionic compounds containing polyatomic ions also involves the same steps as for a binary.
Writing ionic formulas requires knowing the charges of ions in the compound. Get the full course at:. Ask them to indicate whether they know the names of the ions.
Writing a formula for ionic compounds containing polyatomic ions also involves the same steps. Stable ions of atoms in group ia (the first column) of the periodic table form by losing one electron, taking on a +1 charge (usually written as + beside the atom. For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of.
.PNG)

.PNG)







.PNG)
